Hydrogen a Culprit in Battery Degredation
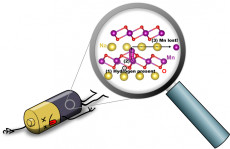
An illustration depicts hydrogen-induced degradation of a sodium-ion battery: (1) When hydrogen is present (circled in black), (2) an Mn atom (purple) can move from the MnO2 layer to the Na layer (yellow); (3) Mn can then move within the Na layer, and will be lost. Image credit: Hartwin Peelaers
Scientific Achievement
UC Santa Barbara computational materials scientist Chris Van de Walle and colleagues, using NERSC, have uncovered a reason for the loss of capacity that occurs over time in sodium batteries: the unintended presence of hydrogen, which leads to degradation of the battery electrode.
Significance and Impact
Batteries power our lives: we rely on them to keep our cell phones and laptops buzzing and our hybrid and electric cars on the road. But ever-increasing adoption of the most commonly used lithium-ion batteries may actually lead to increased cost and potential shortages of lithium — which is why sodium-ion batteries are being researched intensely as a possible replacement. Their performance is good, and sodium, an alkali metal closely related to lithium, is cheap and abundant. The challenge is that sodium-ion batteries have shorter lifetimes than their lithium-based siblings.
Research Details
The calculations are based on hybrid functionals, whose high accuracy comes at a high computational cost, about an order of magnitude more demanding than traditional density functional calculations. Simulations revealed that hydrogen can very easily penetrate the material, and that its presence enables the manganese atoms to break loose from the manganese-oxide backbone that holds the material together. Simulating the barriers for the atomic motion of Mn from the MnO2 layer to the Na layer required finding the minimum path along a trajectory, which required a large number of concurrent cores/nodes.
Ultimately, the calculations allowed the research team to determine that the removal of manganese is irreversible and leads to a decrease in capacity and degradation of the battery. Now that its detrimental impact has been flagged, measures can be taken during fabrication and encapsulation of the batteries to suppress incorporation of hydrogen, which should lead to better performance.
Additional Information
Hydrogen a Culprit in Capacity Loss of Sodium-Ion Batteries
Zhen Zhu, Hartwin Peelaers, Chris Van de Walle, “Hydrogen-Induced Degradation of NaMnO2,” Chemistry of Materials, June 21, 2019, https://doi.org/10.1021/acs.chemmater.9b01458
About NERSC and Berkeley Lab
The National Energy Research Scientific Computing Center (NERSC) is a U.S. Department of Energy Office of Science User Facility that serves as the primary high performance computing center for scientific research sponsored by the Office of Science. Located at Lawrence Berkeley National Laboratory, NERSC serves almost 10,000 scientists at national laboratories and universities researching a wide range of problems in climate, fusion energy, materials science, physics, chemistry, computational biology, and other disciplines. Berkeley Lab is a DOE national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California for the U.S. Department of Energy. »Learn more about computing sciences at Berkeley Lab.







