NERSC Simulations Target ‘Forever Chemicals’ in Soils
MSU researchers use molecular dynamics to reveal new insights into PFAS behavior
July 14, 2022
Contact: cscomms@lbl.gov
This article uses information from a Michigan State University news release.
With the growing awareness about the risks posed by “forever chemicals” in our water, soil, plants, and more, researchers are making new inroads into understanding how these substances function and how we might be able to mitigate their harmful effects. Fortunately, supercomputer simulations are aiding in these efforts.
Formally known as PFAS (perfluoroalkyl and polyfluoroalkyl substances), “forever chemicals” earned the label because they don't break down naturally. Thus when PFAS pollute soil and water, they can enter the food system through plants, livestock, and drinking water. More than 9,000 chemicals qualify as PFAS and they’re found in a wide range of applications, including food packaging, nonstick cookware, and firefighting foams. While time and nature can degrade certain components of these products – and of the waste generated in producing them – the PFAS lingers, accumulating in the environment.
Removing PFAS from soil and water, then, is important for reducing exposure to these chemicals and the harm they can cause, including thyroid disease and increased risk of some cancers.
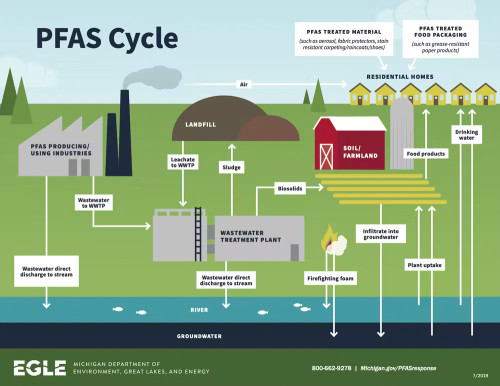
There are many ways PFAS can enter the environment, all of which increase the odds of finding these chemicals in our food or water. (Credit: Michigan Department of Environment, Great Lakes and Energy)
These challenges prompted two Michigan State University (MSU) chemists to use supercomputing resources at the National Energy Research Scientific Computing Center (NERSC) to run simulations that show for the first time how PFAS interact with the clay mineral kaolinite in soil at the molecular level. The researchers, Narasimhan Loganathan and Angela K. Wilson in the College of Natural Science, recently published their findings online in the journal Environmental Science & Technology.
"When you start looking at mitigation strategies, you see a lot about removing PFAS from water, but there's very little about PFAS in soil," said Loganathan, a senior research associate in MSU's Department of Chemistry.
"And some of the (mitigation) studies are 'molecule blind,'" added Wilson, John A. Hannah Distinguished Professor of chemistry and a scientist with the MSU Center for PFAS Research. "That is, they're not paying attention to the chemistry."
Unexpected Findings
Wilson and Loganathan decided to address this gap by performing the first molecular-level simulations of interactions between PFAS with kaolinite. For the study, the duo focused on some of the most prevalent and problematic PFAS chemicals. They chose kaolinite on the soil side because it is a common soil mineral, especially in Michigan.
Working with the molecular dynamics code LAMMPS, commonly used in computational geochemistry, the researchers ran 10 simulation models on the Cori Haswell system at NERSC, along with two simulation models at MSU's Institute for Cyber-Enabled Research. It was a computationally intensive endeavor, Loganathan said. “We were originally allotted 3 million NERSC hours in 2021, but we had to request additional hours to finish this project and another we were working on.” The computer time was allocated by the U.S. Department of Energy's (DOE) Office of Science Basic Energy Sciences program.
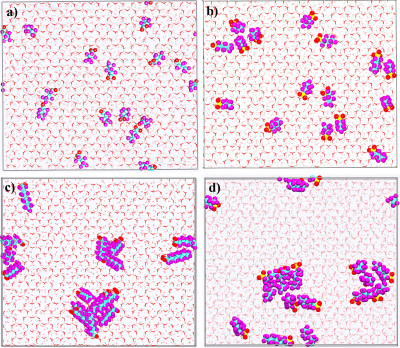
Pictorial representation of PFAS molecules at the external (001) hydroxyl surface of Ca-kaolinite. a) PFBA, b) PFBS, c) PFOA, d) PFOS. Color codes: pink sticks–Al octahedra; red sticks–hydroxyl O; white sticks–hydroxyl H; cyan spheres–C; purple spheres–F; red sphere–O (PFAS); yellow sphere–S. H2O molecules are shown as grayed stick representation. (Credit: Narasimhan Loganathan and Angela K. Wilson, Michigan State University)
The results of the simulations did provide some reasons for optimism with regard to remediation, the team noted. For example, the study found that some PFAS structures had longer carbon chains serving as their backbones congregated on the kaolinite.
"Ideally, this is what you'd want. You'd like all PFAS just to sit in a clump so you can grab it and filter it out," Wilson said. The flipside is that the shorter-chained PFAS were less likely to clump, remaining more mobile in soil. "The take-home message is that not all PFAS behave similarly," she added. "And not all soils behave the same with regard to PFAS."
The simulations are helping the scientific community and others better understand what is happening with the PFAS in soils, Wilson added. “We have been talking with environmental engineers and others who are asking questions about how to best filter PFAS from soil, and some of the knowledge we are getting from this research will be helpful to them.”
"Such molecular level insights are going to be incredibly important for any remediation strategy," Loganathan added.
Wilson’s research team is also examining interactions of PFAS with proteins in the body and studying PFAS in different fish species. The goal, in the soil and biology projects, is to reveal interactions that could help protect more people from PFAS exposure. The protein studies even involve machine learning “shortcuts,” Wilson noted.
"The beauty of computational chemistry is that you can study so many different systems," she said.
NERSC is a DOE Office of Science user facility located at Lawrence Berkeley National Laboratory.
About NERSC and Berkeley Lab
The National Energy Research Scientific Computing Center (NERSC) is a U.S. Department of Energy Office of Science User Facility that serves as the primary high performance computing center for scientific research sponsored by the Office of Science. Located at Lawrence Berkeley National Laboratory, NERSC serves almost 10,000 scientists at national laboratories and universities researching a wide range of problems in climate, fusion energy, materials science, physics, chemistry, computational biology, and other disciplines. Berkeley Lab is a DOE national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California for the U.S. Department of Energy. »Learn more about computing sciences at Berkeley Lab.







