Quarterbacking Catalysts by Positioning Atoms
NERSC Supercomputers Help Scientists Pinpoint Single Oxygen Atoms in Graphene
April 18, 2018
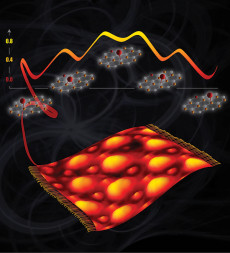
With a graphene sheet thrown like a carpet over the metal, oxygen atoms on top change their binding preference to only one carbon at a time instead of two. Image: Vanda Glezakou and Cortland Johnson, PNNL
To create a winning football team, quarterbacks send their teammates to the right spots. Positioned correctly, the players work around obstacles to drive the ball to the end zone. In much the same way, scientists position catalytic atoms to drive reactions that can yield fuels, plastics, or other desired products.
A team led by Roger Rousseau and Zdenek Dohnálek at Pacific Northwest National Laboratory (PNNL) changed how scientists think about positioning key players: oxygen atoms. These atoms are vital in turning super-thin sheets of carbon, called graphene, into a unique catalytic support.
The team discovered that single oxygen atoms bind to graphene catalytic supports differently than expected. On a freestanding graphene sheet, oxygen most often rests between two carbon atoms. On a graphene sheet resting on a ruthenium metal support, single oxygen atoms bind to just one carbon. Moreover, because the graphene sheet buckles on the metal, these oxygen atoms appear only in certain predictable spots. Their findings were published in the Journal of the American Chemical Society.
"This work let us understand oxygen binding at unprecedented levels," said Rousseau, a PNNL chemist who led the study's theoretical calculations. The team now knows exactly how oxygen atoms bind and the energy involved as well as the influence of the supporting materials.
Designing from the Bottom Up
Creating faster and more efficient catalysts requires designing them from the bottom up. Scientists want to design the right structures to do the job rather than search among countless possibilities. Returning to the football analogy, the quarterback knows what needs to happen and designs the play to get the job done. That's designing the structure for the function. This fundamental research shows scientists how to take advantage of precise spots on the graphene to build up model catalysts that can be faster and more efficient.
"Oxygen atoms on graphene let us bind other groups," said Vanda Glezakou, a theorist on the study. "As a result, they make it possible to design precise and efficient catalytic arrays, essentially positioning the players where they can best work together."
Beginning with a flat piece of ruthenium metal, the scientists grew graphene, a one-atom-thick layer of carbon. The two materials form a superstructure because the carbon and ruthenium atoms do not lay neatly on top of each other. This mismatch causes the graphene to pucker, forcing some carbon atoms to bind to the metal, while others don't. These differences in the graphene binding influence how oxygen atoms bind.
‘Backbreaking’ Calculations
Having created this layered material, they delved into where the oxygen resided and how it behaved. They began with scanning tunneling microscopy. While the instrument is state of the art, the resolution wasn't sufficient to pinpoint the position of static oxygen atoms. So the team heated the material causing the oxygen atoms to move. How the oxygen moved told them about how it was bound.
Analyzing how and why the oxygen atoms moved required density functional theory calculations and massive simulations involving a thousand atoms. For this part of the research, the team used the Edison supercomputer at the National Energy Research Scientific Computing Center (NERSC), a DOE Office of Science User Facility located at Lawrence Berkeley National Laboratory. Their CP2K simulations used about 290,000 CPU hours (roughly 240 CPUs per simulation point). The systems had approximately 1,000 atoms, half of which were metals.
"Metal atoms make simulations even harder, they are slow to converge, and tricky to run the calculations correctly (get the correct state)," Glezakou said. "Because the calculations are so expensive, we have to set up the correct ones, which is not clear a priori, specially when we are trying to validate experimental results."
Matching the graphene/Ru oxide cell dimensions and reproducing the structural pattern (graphene becomes wavy) is not simple and not many people know how to do these calculations so efficiently, she added.
"The data analysis had to be really clever," said Rousseau. "This wasn't something anyone could do. The calculations were backbreaking."
Manh Thuong Nguyen of PNNL, who ran the calculations at NERSC, "was relentless in his pursuit of identifying all the salient structural features that underlie the distinct graphene structure on Ru oxide and the O conformers,” said Glezakou.
By combining laboratory experiments and computational simulations, the team showed that single oxygen atoms bind preferentially to certain carbon atoms. Specifically, carbon atoms that are close to the underlying ruthenium but not bound to it. Less preferred sites for oxygen binding are between two carbon atoms; oxygens bound to carbon atoms that are, in turn, bound to ruthenium; and oxygens on untethered carbon atoms far from the ruthenium.
Having answered how oxygen behaves on carbon, the team is planning to use it as anchors to build model catalysts consisting of single metal atoms and small oxide clusters.
"This research redefines what we know about oxygen binding to carbon atoms on metal-supported graphene, which is very important for their reactivity," said experiment lead Dohnálek, who holds a joint appointment with PNNL and Washington State University.
This article was adapted from resources provided by Pacific Northwest National Laboratory.
About NERSC and Berkeley Lab
The National Energy Research Scientific Computing Center (NERSC) is a U.S. Department of Energy Office of Science User Facility that serves as the primary high performance computing center for scientific research sponsored by the Office of Science. Located at Lawrence Berkeley National Laboratory, NERSC serves almost 10,000 scientists at national laboratories and universities researching a wide range of problems in climate, fusion energy, materials science, physics, chemistry, computational biology, and other disciplines. Berkeley Lab is a DOE national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California for the U.S. Department of Energy. »Learn more about computing sciences at Berkeley Lab.







