Diamond Shines in Molecular Dynamics Simulations
January 6, 2017
Contact: Kathy Kincade, kkincade@lbl.gov, +1 510 495 2124
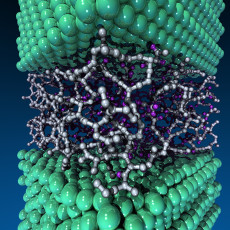
In the tribofilm study, supercomputer simulations allowed researchers from Argonne National Laboratory to virtually test potential catalysts for their “self-healing” properties in a high-temperature, high-pressure engine environment. Visualization: Joseph Insley, Argonne National Laboratory
For centuries diamonds have been revered for their strength, beauty, value and utility. Now a team of researchers from Argonne National Laboratory, running molecular dynamics calculations at the Argonne Leadership Computing Facility (ALCF) and Berkeley Lab’s National Energy Research Scientific Computer Center (NERSC), are finding additional reasons to celebrate this complex material—and it has nothing to do with color, cut or clarity.
In a series of papers published in in Science, Nature and Nature Communications, experimentalists and computational scientists from Argonne’s Center for Nanoscale Materials (CNM) shared several “firsts” in their ongoing efforts to uncover new characteristics in diamond and diamond-like carbons that make these materials even more attractive, particularly for industrial applications.
For example, the Nature study, published in August 2016, highlights their discovery of a revolutionary diamond-like film that is generated by the heat and pressure of an automotive engine. This ultra-durable, self-lubricating tribofilm (a film that forms between moving surfaces) could have profound implications for the efficiency and durability of future engines and other moving metal parts that can be made to develop self-healing, diamond-like carbon tribofilms.
The phenomenon was first discovered several years ago through experiments conducted by researchers in the Tribology and Thermal-Mechanics Department in Argonne's Center for Transportation Research. But it took theoretical insight using supercomputing resources to fully understand what was happening at the molecular level in the experiments. Argonne nanoscientist Subramanian Sankaranarayanan and postdoctoral researcher Badri Narayanan ran molecular dynamics simulations on Argonne’s Mira system and NERSC’s Edison system to understand what was happening at the atomic level. These calculations helped them determine that the catalyst metals in the nanocomposite coatings were stripping hydrogen atoms from the hydrocarbon chains of the lubricating oil, then breaking the chains down into smaller segments. The smaller chains then joined together under pressure to create the highly durable DLC tribofilm.
“This is an example of catalysis under extreme conditions created by friction. It is opening up a new field where you are merging catalysis and tribology, which has never been done before,” said Sankaranarayanan. “This new field of tribocatalysis has the potential to change the way we look at lubrication.”
In the Nature Communications study, published in July 2016, a team of Argonne and University of California-Riverside researchers once again used a combination of experiments and molecular dynamics simulations to demonstrate how diamond—in this case ultrananocrystalline diamond that serves as a substrate—can be used to grow graphene that contains relatively few impurities and costs less to make, in shorter time and at lower temperatures compared to the process widely used to make graphene today. Current graphene fabrication protocols introduce impurities during the etching process itself, which involves adding acid and extra polymers, and when they are transferred to a different substrate for use in electronics. These impurities negatively affect the electronic properties of the graphene, the researchers noted.
The simulations—which were developed by Sankaranarayanan and his post-docs, Badri Narayanan and Sanket Deshmukh, and utilized 300,000 to 500,000 node hours at NERSC in addition to computing time at Argonne—helped the team understand the molecular-level processes underlying graphene growth. They ran three different sets of calculations on NERSC’s Edison supercomputer to tease out the sequence of events leading to graphene nucleation on nickel and to determine what kind of graphene structures can grow on different crystal orientations.
“NERSC is a very good resource to have because it allows the flexibility to do intermediate, production-run calculations,” Sankaranarayanan said. “In this example, you have a lot of things happening mechanistically, and the experimentalists have an end point and the time scales involved are quite fast. But they have not yet reached a stage where in situ experiments can be performed on these kinds of rapidly evolving interfaces, and they want to understand the dynamics of what is happening at the nanosecond and microsecond time scales. It is this dynamical evolution that the experimentalists want us to simulate.”
In an earlier, related study published in Science, the Argonne team described how a series of molecular dynamics simulations paved the way for the design of a near-frictionless hybrid material. The research team again used a combination of experiments and simulations to demonstrate that superlubricity can be realized at engineering scale when graphene is used in combination with nanodiamond particles and diamond-like carbon. Considering that nearly one-third of every fuel tank is spent overcoming friction in automobiles, a material that can achieve superlubricity would greatly benefit industry and consumers alike.
“The beauty of this particular discovery is that we were able to see sustained superlubricity at the macroscale for the first time, proving this mechanism can be used at engineering scales for real-world applications,” Sankaranarayanan said. “It was really a big breakthrough that purely came out of calculations that we did initially at NERSC and then at ACLF.”
About NERSC and Berkeley Lab
The National Energy Research Scientific Computing Center (NERSC) is a U.S. Department of Energy Office of Science User Facility that serves as the primary high performance computing center for scientific research sponsored by the Office of Science. Located at Lawrence Berkeley National Laboratory, NERSC serves almost 10,000 scientists at national laboratories and universities researching a wide range of problems in climate, fusion energy, materials science, physics, chemistry, computational biology, and other disciplines. Berkeley Lab is a DOE national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California for the U.S. Department of Energy. »Learn more about computing sciences at Berkeley Lab.







