A First: Trapping Noble Gases in 2D Porous Structures at Room Temp
Simulations at NERSC Confirm Surprising Experimental Breakthrough
August 21, 2017
Contact: Kathy Kincade, kkincade@lbl.gov, +1 510 495 2124
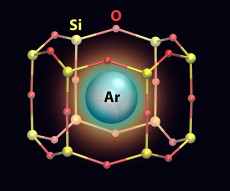
An artistic rendering of an argon (Ar) atom trapped in a nanocage that has a silicon (Si)-oxygen (O) framework.
A materials science breakthrough at the nanoscale could lead to better methods for capturing noble gases, such as radioactive krypton and xenon generated by nuclear power plants.
Scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory had just finished an experiment with a two-dimensional (2D) structure they synthesized for catalysis research when, to their surprise, they discovered that atoms of argon gas had become trapped inside the structure’s nanosized pores. Argon and other noble gases have previously been trapped in three-dimensional (3D) porous materials, but immobilizing them on surfaces had only been achieved by cooling the gases to very low temperatures to condense them or accelerating gas ions to implant them directly into materials—processes that can be energy intensive and expensive.
With this new breakthrough, scientists will be able to use traditional surface-science tools—such as x-ray photoelectron and infrared reflection absorption spectroscopy—to perform detailed studies of single gas atoms in confinement. Understanding how individual atoms enter and exit the nanoporous frameworks could help scientists design new materials for gas separation and nuclear waste remediation.
“We are the first team to trap a noble gas in a 2D porous structure at room temperature,” said Anibal Boscoboinik, a materials scientist at Brookhaven Lab’s Center for Functional Nanomaterials (CFN), a DOE Office of Science User Facility where part of the research was conducted.
Calculations run at the National Energy Research Scientific Computing Center (NERSC), a DOE Office of Science User Facility located at Lawrence Berkeley National Laboratory, helped corroborate the research team’s findings, which were reported July 17 in Nature Communications.
2D Zeolite Models
For the study, scientists from Brookhaven Lab, Stony Brook University and the National University of San Luis in Argentina synthesized 2D aluminosilicate (composed of aluminum, silicon and oxygen) films on top of a ruthenium metal surface. They created this 2D model catalyst material to study the chemical processes that occur in the industrially used 3D catalyst (called a zeolite), which has a cage-like structure with open pores and channels the size of small molecules. Because the catalytically active surface is enclosed within these cavities, it is difficult to probe with traditional surface-science tools. The 2D analogue material has the same chemical composition and active site as the 3D porous zeolite, but its active site is exposed on a flat surface, which is easier to access with such tools.
To confirm that the argon atoms were trapped in these “nanocages,” the scientists exposed the 2D material to argon gas and measured the kinetic energy and number of electrons ejected from the surface after striking it with an x-ray beam. They performed these studies at the former National Synchrotron Light Source I (NSLS-I) and its successor facility, NSLS-II, both DOE Office of Science User Facilities at Brookhaven. Because the binding energies of core electrons are unique to each chemical element, the resulting spectra reveal the presence and concentration of elements on the surface. In a separate experiment conducted at the CFN, they grazed a beam of infrared light over the surface while introducing argon gas. When atoms absorb light of a specific wavelength, they undergo changes in their vibrational motions that are specific to that element’s molecular structure and chemical bonds.
Simulations Run on Edison, Cori
After studying adsorption, the scientists examined the reverse process of desorption by incrementally increasing the temperature until the argon atoms completely released from the surface at 350 degrees Fahrenheit. They corroborated their experimental spectra with theoretical calculations of the amount of energy associated with argon entering and leaving the cages. Second author Mengen Wang, a student in Deyu Lu’s group at Brookhaven, ran a series of 60 VASP calculations on NERSC’s Edison and Cori supercomputers, using a total of 288 cores and 622,080 CPU hours. VASP (Vienna Ab Initio Simulation Package) is a code widely used in materials science research.
“The simulations—specifically first principles modeling—in this project played multiple roles,” Lu said. “Since so far experimentally it is very difficult to ‘see’ individual argon atoms directly, theory plays the key role of ‘microscope’ that can tell where the argon atom wants to go based on quantum mechanical laws. Our calculations clearly show that the argon atom prefers the trapping site inside the cage over the interfacial site, and these results are full consistent with the X-ray photoelectron spectroscopy measurements.”
Theory and computation also provide insights on the mechanism of the adsorption and desorption, he added. The calculated adsorption and desorption barriers can be used to obtain the rates that are necessary to describe such processes.
“Theory is crucial not just for interpreting experimental results but also for linking all the experimental evidence together in a constructive way to form a coherent understanding of this fascinating system,” Lu said.
While their main goal going forward is to continue investigating zeolite catalytic processes on the 2D material, the scientists are interested in learning the impact of different pore sizes on the materials’ ability to trap and filter gas molecules.
“As we seek to better understand the material, interesting and unexpected findings keep coming up,” said Boscoboinik. “The ability to use surface-science methods to understand how a single atom of gas behaves when it is confined in a very small space opens up lots of interesting questions for researchers to answer.”
This article used materials provided by Brookhaven National Laboratory.
About NERSC and Berkeley Lab
The National Energy Research Scientific Computing Center (NERSC) is a U.S. Department of Energy Office of Science User Facility that serves as the primary high performance computing center for scientific research sponsored by the Office of Science. Located at Lawrence Berkeley National Laboratory, NERSC serves almost 10,000 scientists at national laboratories and universities researching a wide range of problems in climate, fusion energy, materials science, physics, chemistry, computational biology, and other disciplines. Berkeley Lab is a DOE national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California for the U.S. Department of Energy. »Learn more about computing sciences at Berkeley Lab.







