Hydrogen a Culprit in Capacity Loss of Sodium-Ion Batteries
NERSC’s computing resources reveal a trigger for degradation of battery electrodes
August 1, 2019
Adapted from a UC Santa Barbara press release.
Batteries power our lives: we rely on them to keep our cell phones and laptops buzzing and our hybrid and electric cars on the road. But ever-increasing adoption of the most commonly used lithium-ion batteries may actually lead to increased cost and potential shortages of lithium — which is why sodium-ion batteries are being researched intensely as a possible replacement. Their performance is good, and sodium, an alkali metal closely related to lithium, is cheap and abundant. The challenge is that sodium-ion batteries have shorter lifetimes than their lithium-based siblings.
UC Santa Barbara computational materials scientist Chris Van de Walle and colleagues, using the National Energy Research Scientific Computing Center (NERSC) at Lawrence Berkeley National Laboratory, have now uncovered a reason for the loss of capacity that occurs over time in sodium batteries: the unintended presence of hydrogen, which leads to degradation of the battery electrode. Van de Walle and co-authors Zhen Zhu and Hartwin Peelaers published their findings in the journal Chemistry of Materials.
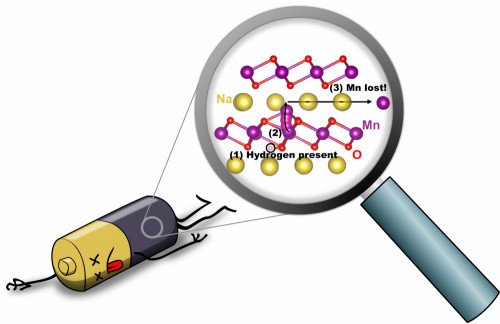
An illustration depicts hydrogen-induced degradation of a sodium-ion battery: (1) When hydrogen is present (circled in black), (2) an Mn atom (purple) can move from the MnO2 layer to the Na layer (yellow); (3) Mn can then move within the Na layer, and will be lost. Image credit: Hartwin Peelaers
“Hydrogen is commonly present during the fabrication of the cathode material, or it can be incorporated from the environment or from the electrolyte,” said Zhu, who is now at Google. “Hydrogen is known to strongly affect the properties of electronic materials, so we were curious about its effect on NaMnO2, a common cathode material for sodium-ion batteries.” To study this, the researchers used computational techniques that are capable of predicting the structural and chemical effects that arise from the presence of impurities.
Professor Peelaers (now at the University of Kansas) noted that “such accurate calculations are computationally very demanding, so the use of NERSC’s supercomputing facility was essential to perform these calculations. The calculations are based on hybrid functionals, whose high accuracy comes at a high computational cost, about an order of magnitude more demanding than traditional density functional calculations. We used the Haswell nodes on Cori. The performant processors, combined with the fast interconnects, enabled us to fully parallelize our calculations, leading to a significant speedup. We quickly realized that hydrogen can very easily penetrate the material, and that its presence enables the manganese atoms to break loose from the manganese-oxide backbone that holds the material together. Simulating the barriers for the atomic motion of Mn from the MnO2 layer to the Na layer required finding the minimum path along a trajectory, which required a large number of concurrent cores/nodes.“
Ultimately, the calculations allowed the research team to determine that the removal of manganese is irreversible and leads to a decrease in capacity and degradation of the battery.
“Earlier research had shown that loss of manganese could take place at the interface with the electrolyte or could be associated with a phase transition, but it did not really identify a trigger,” said Van de Walle, in whose Computational Materials Group the studies were performed. “Our new results show that the loss of manganese can occur anywhere in the material, if hydrogen is present. Because hydrogen atoms are so small and reactive, hydrogen is a common contaminant in materials. Now that its detrimental impact has been flagged, measures can be taken during fabrication and encapsulation of the batteries to suppress incorporation of hydrogen, which should lead to better performance.”
In fact, the researchers suspect that even the ubiquitous lithium-ion batteries may suffer from the ill effects of unintended hydrogen incorporation. Whether this causes fewer problems because fabrication methods are more advanced in this more mature materials system, or because there is a more fundamental reason for the lithium batteries to be more resistant to hydrogen is not clear at present, and will be an area of future research.
NERSC is a DOE Office of Science User Facility.
Chris G. Van de Walle can be reached at vandewalle@mrl.ucsb.edu.
About NERSC and Berkeley Lab
The National Energy Research Scientific Computing Center (NERSC) is a U.S. Department of Energy Office of Science User Facility that serves as the primary high performance computing center for scientific research sponsored by the Office of Science. Located at Lawrence Berkeley National Laboratory, NERSC serves almost 10,000 scientists at national laboratories and universities researching a wide range of problems in climate, fusion energy, materials science, physics, chemistry, computational biology, and other disciplines. Berkeley Lab is a DOE national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California for the U.S. Department of Energy. »Learn more about computing sciences at Berkeley Lab.







