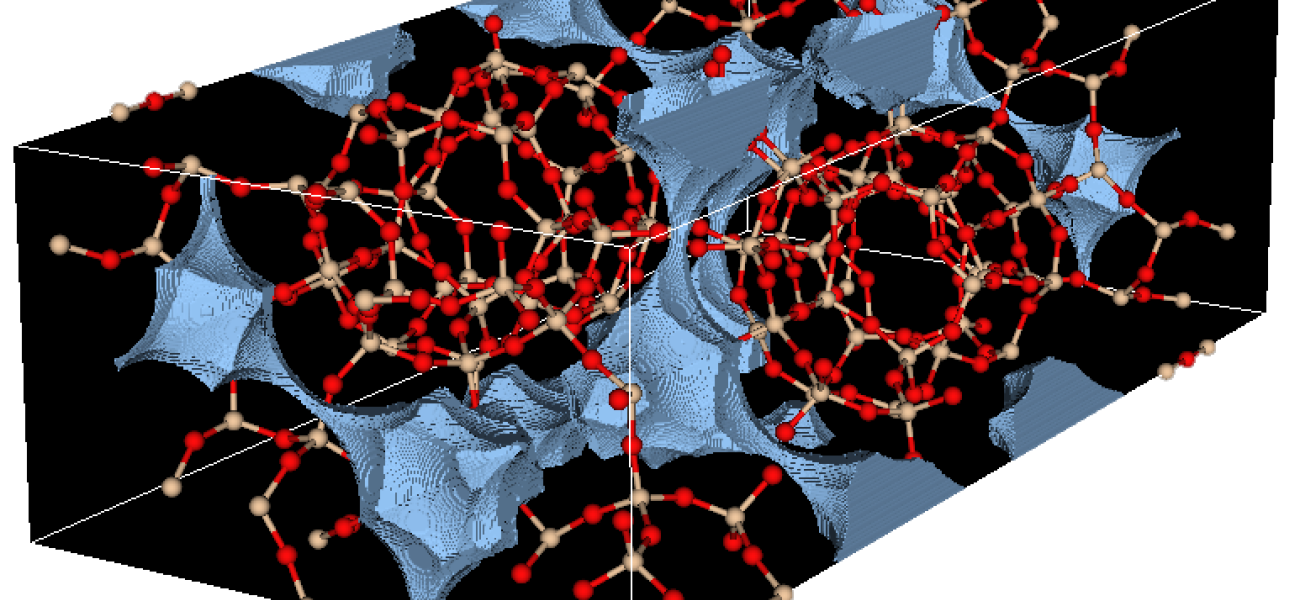
Zeolites are porous minerals with large internal surface area. - Credit: Maciej Haranczyk, Berkeley Lab
Science breakthrough
With support from the Perlmutter supercomputer at the National Energy Research Scientific Computing Center (NERSC), scientists have tackled a long-standing problem in industrial chemistry. They have created a catalyst that converts methane and carbon dioxide into a useful chemical product with excellent long-term stability at high temperatures. Their work was published in Nature Communications in October.
Science background
Through a process known as dry reforming of methane (DRM), nickel-based catalysts have long been used to convert methane and carbon dioxide into syngas, a mixture of hydrogen and carbon monoxide widely used as a base ingredient for everything from diesel fuel to fertilizer. However, common catalysts that make the chemical reaction possible are quickly deactivated by the high temperatures needed for the reaction to occur. This is because carbon atoms left over from the reaction form coke, a form of pure carbon, that covers the reactive surface so that it can no longer participate in the reaction.
Researchers developed a new method to synthesize a catalyst that sidesteps these complications by incorporating zeolite, a porous mineral whose large internal surface area helps stabilize the nickel surfaces where the reaction occurs, keeping them free of coke and able to continue reacting. With this zeolite-based catalyst, the process becomes much more energy-efficient and turns two greenhouse gases into commercially valuable syngas. The method may also be valuable for creating catalysts for other applications, making this discovery a possible starting point and an achievement in its own right.
Science breakdown
The researchers used Perlmutter and GPU-accelerated VASP (Vienna Ab Initio Simulation Package) to determine the stable state of the nickel catalyst inside the zeolite framework.
They noted that they were impressed by the GPU acceleration after some initial trial-and-error in transitioning from CPU to GPU for VASP calculations of catalyst structures and catalytic mechanisms.
Research lead
Junyan Zhang, Felipe Polo-Garzon
Co-authors
Y. Li, H. Song, L. Zhang, Y. Wu, Y. He, L. Ma, J. Hong, A. Tayal, N. Marinkovic, D. Jiang, Z. Li, Zi Wu
Publication
Zhang, J., Li, Y., Song, H. et al. Tuning metal-support interactions in nickel–zeolite catalysts leads to enhanced stability during dry reforming of methane. Nat Commun 15, 8566 (2024). https://doi.org/10.1038/s41467-024-50729-8
Funding
U.S. Department of Energy Office of Science, Office of Basic Energy Sciences
User facilities
NERSC
About NERSC and Berkeley Lab
The National Energy Research Scientific Computing Center (NERSC) is the mission computing facility for the U.S. Department of Energy Office of Science, the nation’s single largest supporter of basic research in the physical sciences.
Located at Lawrence Berkeley National Laboratory (Berkeley Lab), NERSC serves 11,000 scientists at national laboratories and universities researching a wide range of problems in climate, fusion energy, materials sciences, physics, chemistry, computational biology, and other disciplines. An average of 2,000 peer-reviewed science results a year rely on NERSC resources and expertise, which has also supported the work of seven Nobel Prize-winning scientists and teams.
NERSC is a U.S. Department of Energy Office of Science User Facility.
Media contact: Email our communications team ⟶