Coupling Experiments to Theory to Build a Better Battery
Molecular Dynamics Simulations Confirm Viability of New Polymer Binder
January 18, 2018
By Laurie Chong
Contact: cscomms@lbl.gov
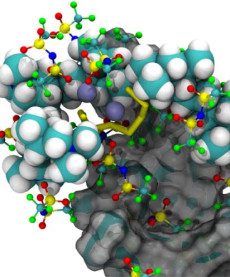
This illustration shows the formation of complex ion clusters during the cycling of a lithium-sulfur battery cell. The clusters consist of cationic polymer binders, battery electrolyte, and anionic sulfur-active materials. Credit: Berkeley Lab
Lithium-sulfur batteries are promising candidates for replacing common lithium-ion batteries in electric vehicles since they are cheaper, weigh less, and can store nearly double the energy for the same mass. However, lithium-sulfur batteries become unstable over time, and their electrodes deteriorate, limiting widespread adoption.
Now, a team of researchers led by scientists at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) have reported that a new lithium-sulfur battery component allows a doubling in capacity compared to a conventional lithium-sulfur battery, even after more than 100 charge cycles at high current densities, which are key performance metrics for their adoption in electric vehicles (EVs) and in aviation.
They did it by designing a new polymer binder that actively regulates key ion transport processes within a lithium-sulfur battery, and have also shown how it functions on a molecular level. The work was recently reported in the journal Nature Communications.
“The new polymer acts as a wall,” said Brett Helms, a staff scientist at Berkeley Lab’s Molecular Foundry and corresponding author of the study. “The sulfur is loaded into the pores of a carbon host, which are then sealed by our polymer. As sulfur participates in the battery’s chemical reactions, the polymer prevents the negatively charged sulfur compounds from wandering out. The battery has great promise for enabling the next generation of EVs.”
When a lithium-sulfur battery stores and releases energy, the chemical reaction produces mobile molecules of sulfur that become disconnected from the electrode, causing it to degrade and ultimately lowering the battery’s capacity over time. To make these batteries more stable, researchers have traditionally worked to develop protective coatings for their electrodes, and to develop new polymer binders that act as the glue holding battery components together. These binders are intended to control or mitigate the electrode’s swelling and cracking.
The new binder goes a step further. Researchers from the Organic Synthesis Facility at Berkeley Lab’s Molecular Foundry, a research center specializing in nanoscale science, designed a polymer to keep the sulfur in close proximity to the electrode by selectively binding the sulfur molecules, counteracting its migratory tendencies.
The next step was to understand the dynamic structural changes that are likely to occur during charging and discharging as well as at different states of charge. David Prendergast, who directs the Foundry’s Theory Facility, and Tod Pascal, a project scientist in the Theory Facility, built a simulation to test the researchers’ hypotheses about the polymer’s behavior.
“We can now reliably and efficiently model sulfur chemistry within these binders based on learning from detailed quantum mechanical simulations of the dissolved sulfur-containing products,” stated Prendergast.
Their large-scale molecular dynamics simulations, conducted on supercomputing resources at Berkeley Lab’s National Energy Research Scientific Computing Center (NERSC), confirmed that the polymer has an affinity for binding the mobile sulfur molecules, and also predicted that the polymer would likely show a preference for binding different sulfur species at different states of charge for the battery. Experiments conducted at Berkeley Lab’s Advanced Light Source and Argonne National Laboratory’s Electrochemistry Discovery Lab confirmed these predictions.
The research team took their study one step further by also examining the performance of lithium-sulfur cells made with the new polymer binder. Through a set of experiments, they were able to analyze and quantify how the polymer affects the chemical reaction rate in the sulfur cathode, which is key to achieving high current density and high power with these cells.
By nearly doubling the battery’s electrical capacity over long-term cycling, the new polymer raises the bar on the capacity and power of lithium-sulfur batteries.
The combined understanding of the synthesis, theory, and characteristics of the new polymer have made it a key component in the prototype lithium-sulfur cell at DOE’s Joint Center for Energy Storage Research (JCESR).
Berkeley Lab’s Molecular Foundry, Advanced Light Source and NERSC are DOE Office of Science User Facilities that are open to visiting researchers from around the nation and world.
Researchers from JCESR at Berkeley Lab and Argonne National Lab comprised the team, together with scientists from the Massachusetts Institute of Technology, and UC Berkeley. Funding for the project was provided by JCESR, a Department of Energy Innovation Hub that is supported by the DOE Office of Science.
About NERSC and Berkeley Lab
The National Energy Research Scientific Computing Center (NERSC) is a U.S. Department of Energy Office of Science User Facility that serves as the primary high performance computing center for scientific research sponsored by the Office of Science. Located at Lawrence Berkeley National Laboratory, NERSC serves almost 10,000 scientists at national laboratories and universities researching a wide range of problems in climate, fusion energy, materials science, physics, chemistry, computational biology, and other disciplines. Berkeley Lab is a DOE national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California for the U.S. Department of Energy. »Learn more about computing sciences at Berkeley Lab.







